Introduction
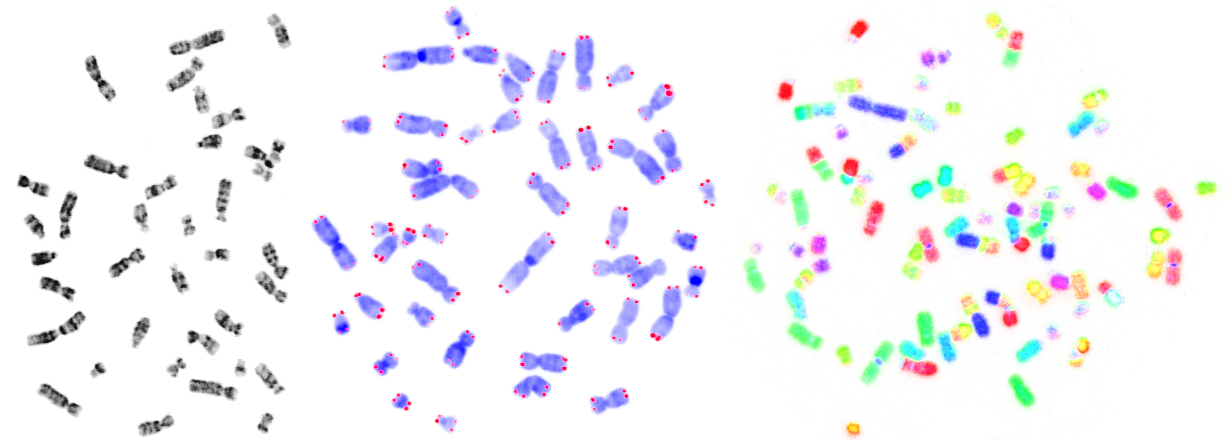
My research interests focus primarily on the capping structure at the end of eukaryotic chromosomes, called telomere. I am particularly interested in the phenomenon of telomere shortening and its implications for human disease, aging and evolution. The telomere story goes back to the Nobel laureate Barbara McClintock. She did some milestone experiments in the thirties, showing that linear chromosomes without telomeres are unstable and result in chromosomal aberrations. In 1961, Leonard Hayflick discovered the limited replication potential of human cells. Ten years later, Alexey Olovnikov proposed that this 'mitotic clock' results from the end replication problem of linear DNA molecules, which leads to replicative telomere erosion.
Today, we know that telomeres erode in somatic tissues during life, and short telomeres are thought to be causally involved in the aging phenotype. However, according to mainstream theories, telomeres remain stable in the germline of a species...and this is the part, where I completely disagree with the mainstream:
The species clock hypothesis (2004) is based on the idea that telomeres shorten not only in somatic cells during a lifetime of an individual, but in the germline between generations of a species. This biological clock would limit the lifetime of every species and would therefore represent an intrinsic cause of extinction (and speciation).
In 2014, I further developed the theory and published the telomeric sync model of speciation, which provides a biological framework for the old European model of saltatory evolution of nonadaptive characters.